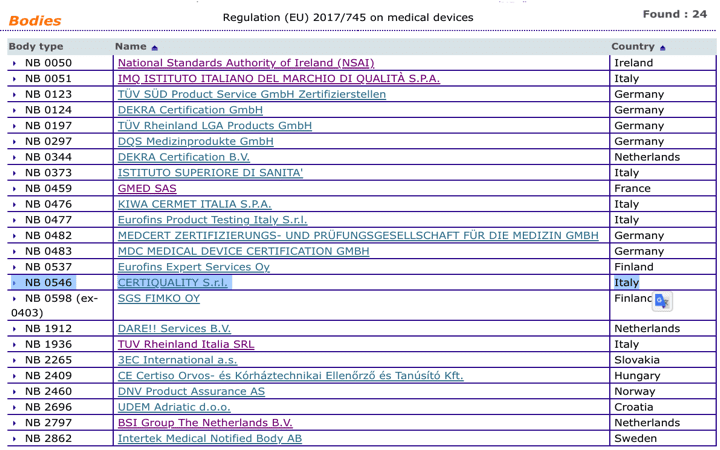
Organismos Notificados MDR (24): CERTIQUALITY (Italia) ON num. 0546 nuevo ON. Enhorabuena!!! | Red de Tecnologías Sanitarias y Productos Sanitarios

CE 0197 Reinforced Arterial Catheter - China Medical Device Manufacturer, Catheter | Made-in-China.com

Organismos Notificados: TÜV Rheinland LGA Products ( @tuv_es ) NB num. 0197 nuevo ON con IVDR. Enhorabuena!!!

Which EU Notified Bodies Have Been “Designated” Under the MDR 2017/745 and IVDR 2017/746? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
Transition to EU IVD Regulation (EU) 2017/746 and considerations for non-EU regulatory authorities on managing the impact to pro