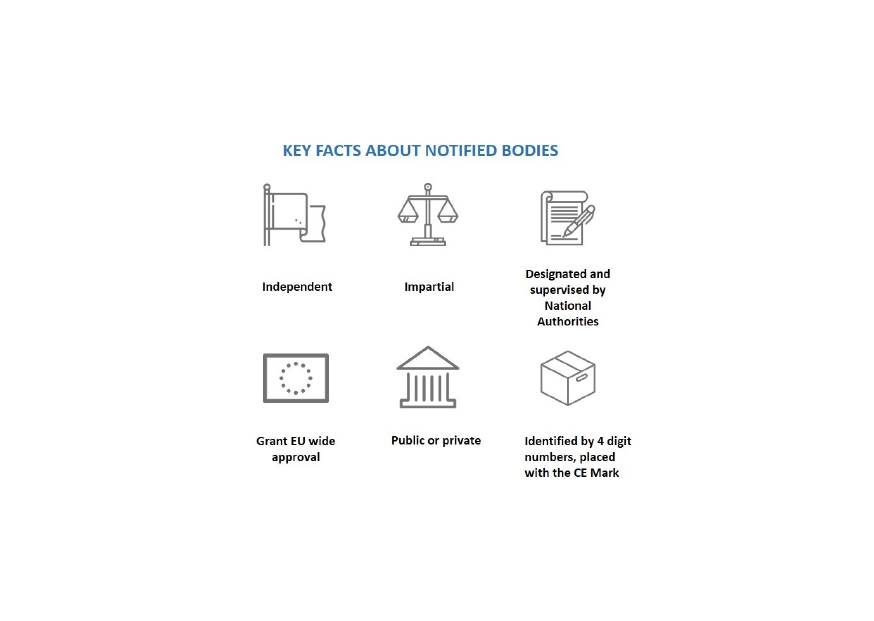
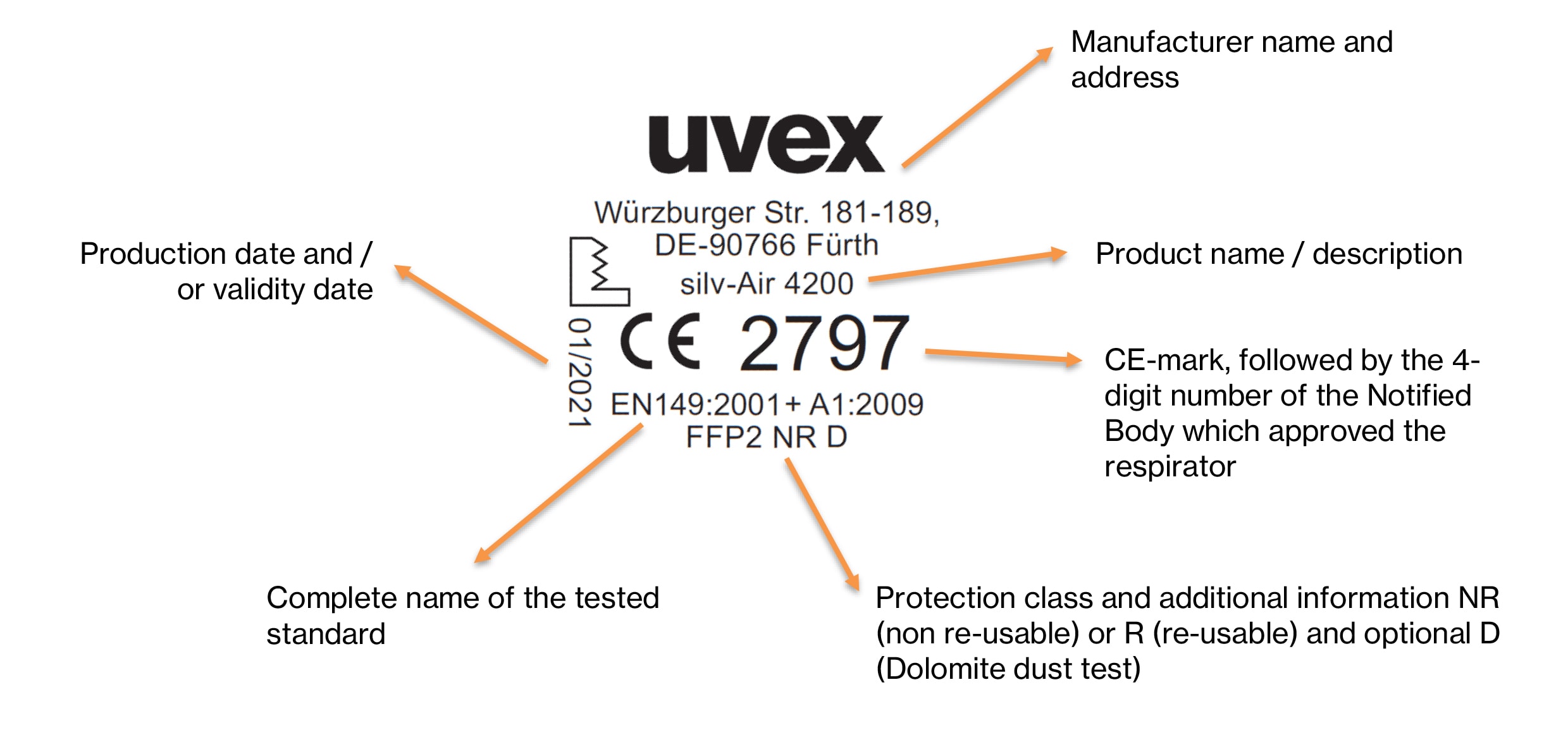
Organismos Notificados: BSI NL (ON num.2797 @BSI_NL ) y DARE!! (ON num.1912 @CEMarkering ) nuevos Organismos Notificados con el reglamento MDR – Enhorabuena!!

Which EU Notified Bodies Have Been “Designated” Under the MDR 2017/745 and IVDR 2017/746? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog

Organismos Notificados: BSI NL (ON num.2797 @BSI_NL ) y DARE!! (ON num.1912 @CEMarkering ) nuevos Organismos Notificados con el reglamento MDR – Enhorabuena!!

CE Marking a Medical Device under the EU MDR | Wellcome / EPSRC Centre for Interventional and Surgical Sciences - UCL – University College London

EC Certificate - Full Quality Assurance System: Directive 93/42/EEC On Medical Devices, Annex II Excluding Section 4 | PDF | Medical Device | Business

Otohub Srl on X: "#Otohub announced today that its entire current products line (#OtoPad, #OtoRemote and #OtoKiosk) has earned CE Mark certification from the British Standards Institution (#BSI). 👉 https://t.co/ZzZCh7TaRg #CEmark #ECcertificate #

Team-NB publica el position paper «recommendations on the classification of devices intended to detect the presence or the exposure to SARS-CoV-2» cambio clase D a clase C | Red de Tecnologías Sanitarias