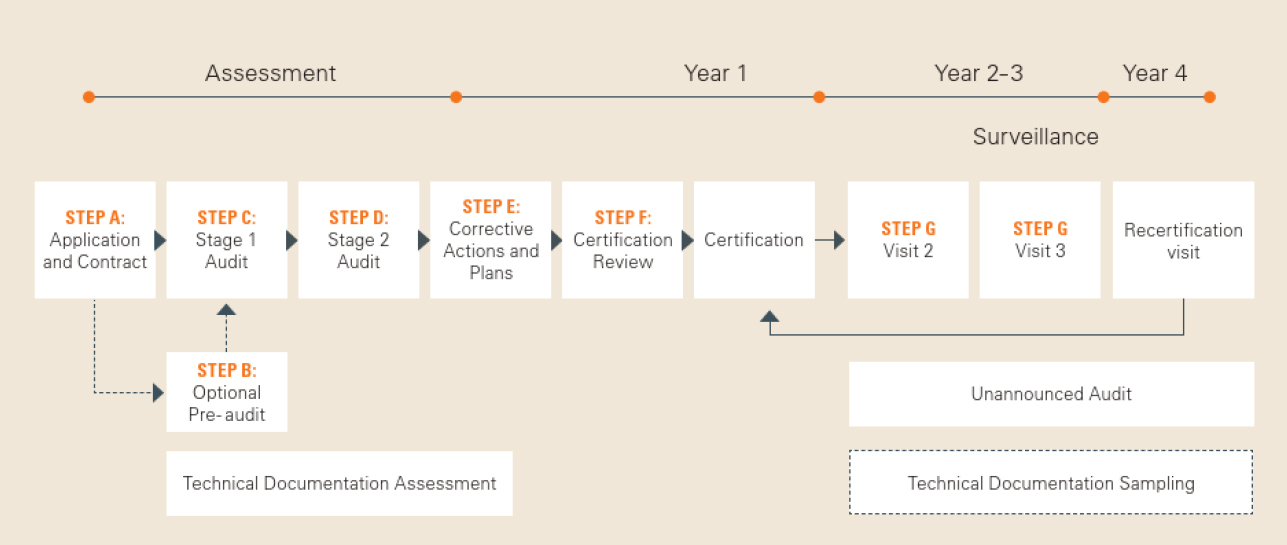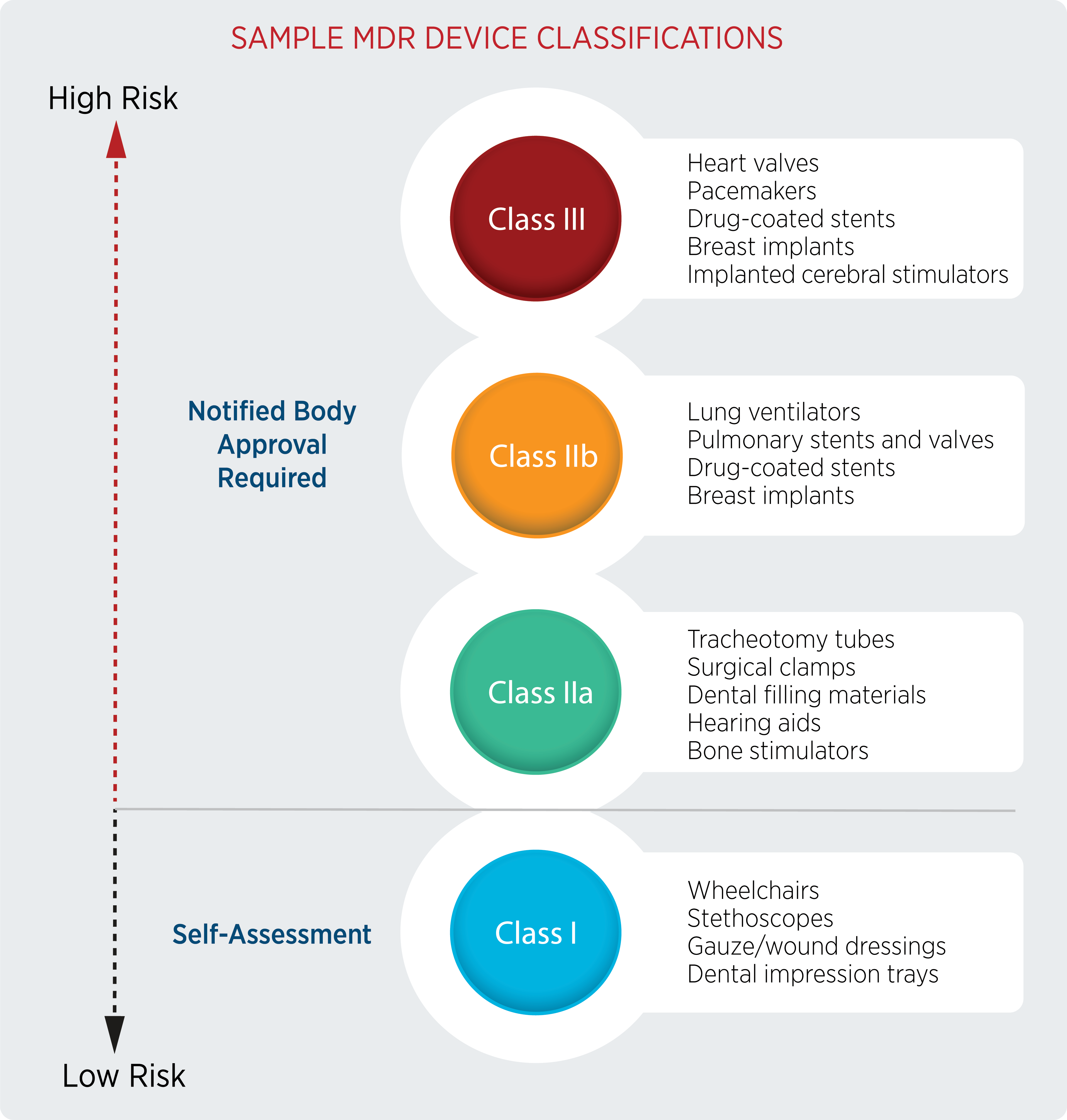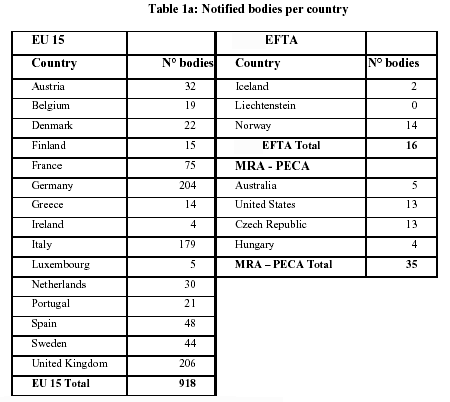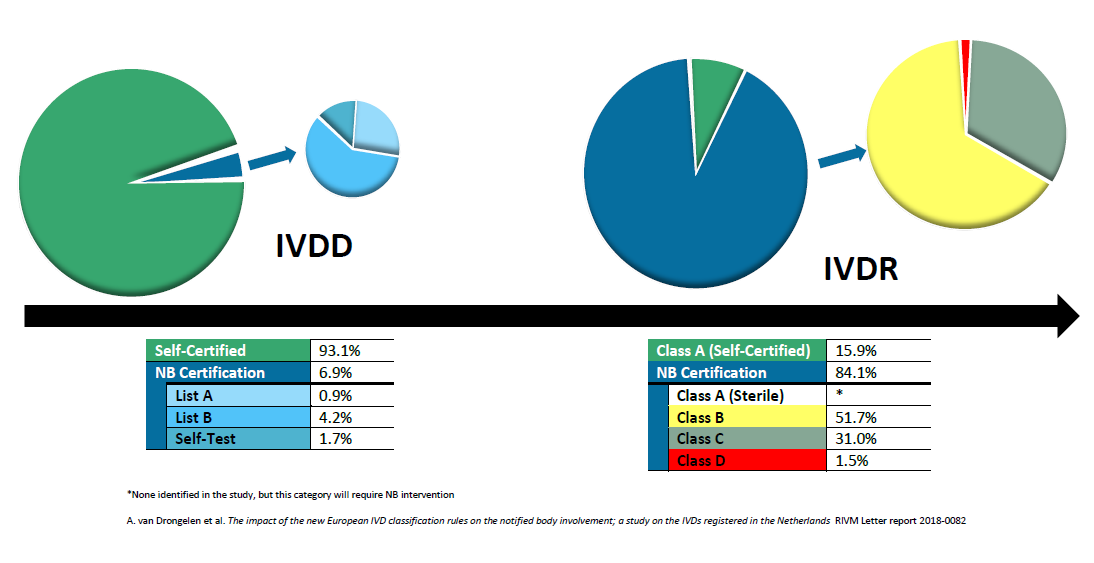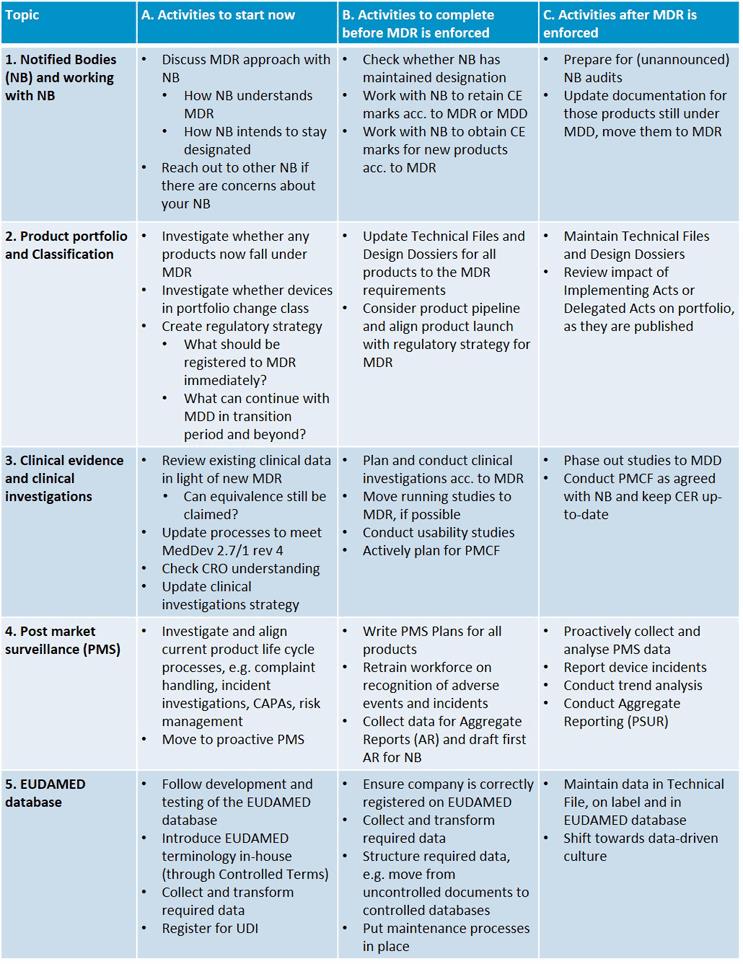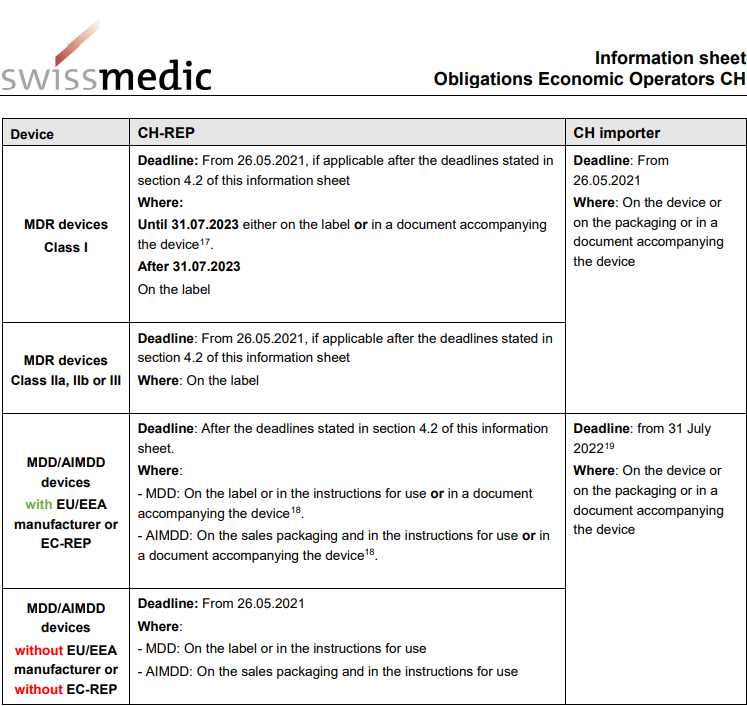
MDR: 26 Notified Bodies on NANDO & Swiss economic operator's requirements updated! · MDlaw – Information platform on European medical device regulations
Availability and capacity of notified bodies to carry out conformity assessments for COVID-19 related medical devices and in vit