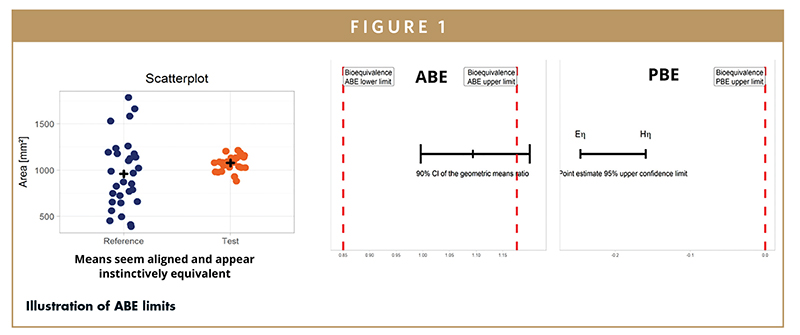
NASAL SPRAY BIOEQUIVALENCE - Between-Batch Bioequivalence (BBE): An Alternative Statistical Method to Assess In Vitro Bioequivalence of Nasal Product

Bioequivalence for locally acting nasal spray and nasal aerosol products: standard development and generic approval. - Abstract - Europe PMC

FDA approves Narcan nasal spray for opioid overdoses, hopes for wider, safer use | Healthcare Finance News

Tests recommended for nasal drug product characterization/development... | Download Scientific Diagram

Statement on FDA Decision to Delay New Epinephrine Nasal Spray for Anaphylaxis | Allergy & Asthma Network