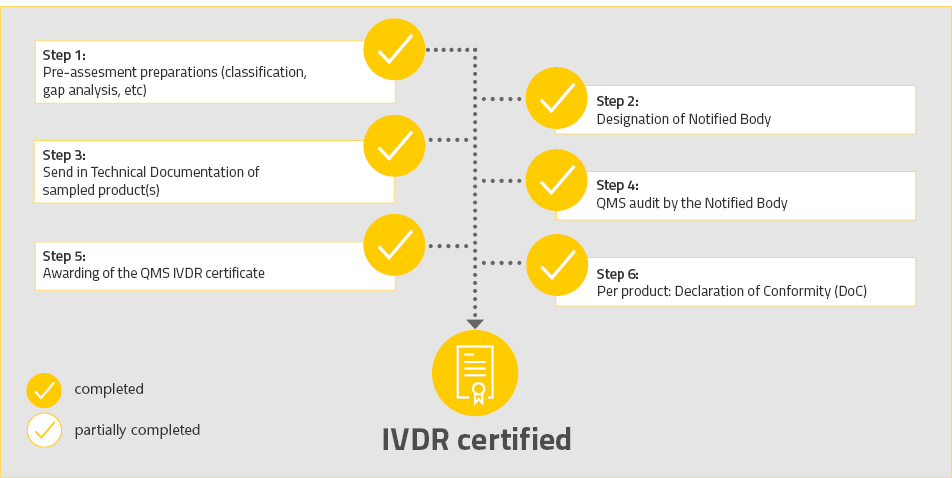
Lunch & Learn: How to best interact with a Notified Body for a successful MDR/IVDR application? - KI Science Park

Which EU Notified Bodies Have Been “Designated” Under the MDR 2017/745 and IVDR 2017/746? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
Organismos Notificados MDR (44): RISE – Medical Notified Body AB (Suecia) ON num. 3033 nuevo Organismo Notificado. Enhorabuena !!!

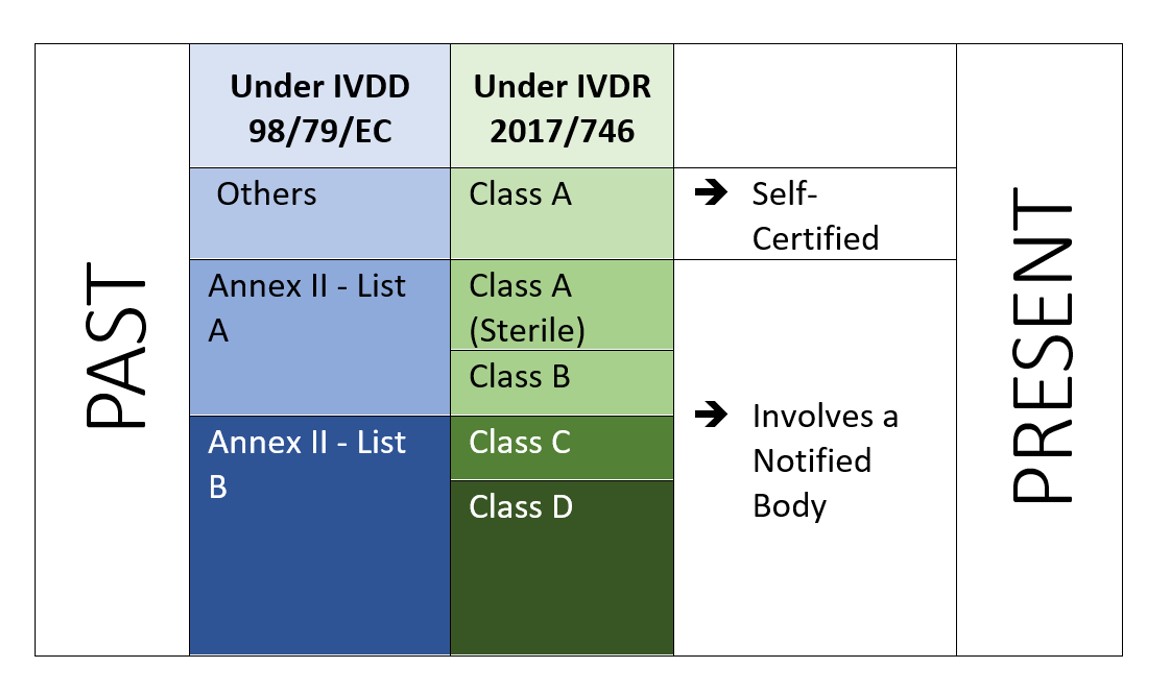
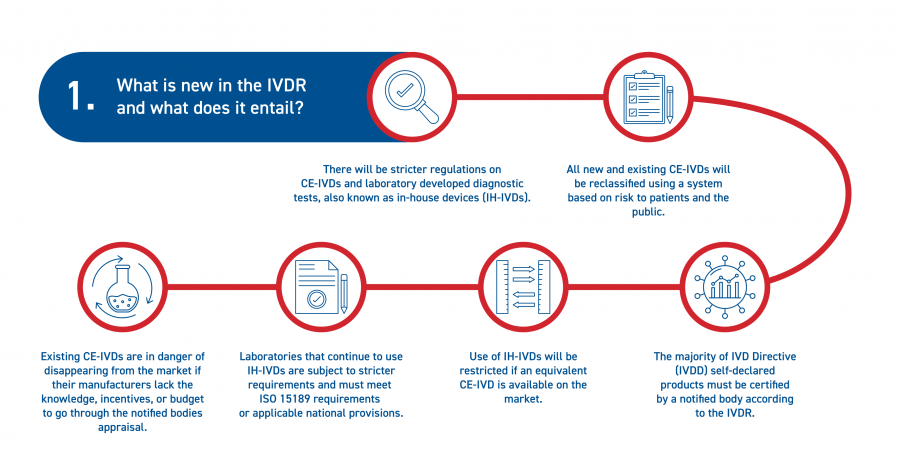
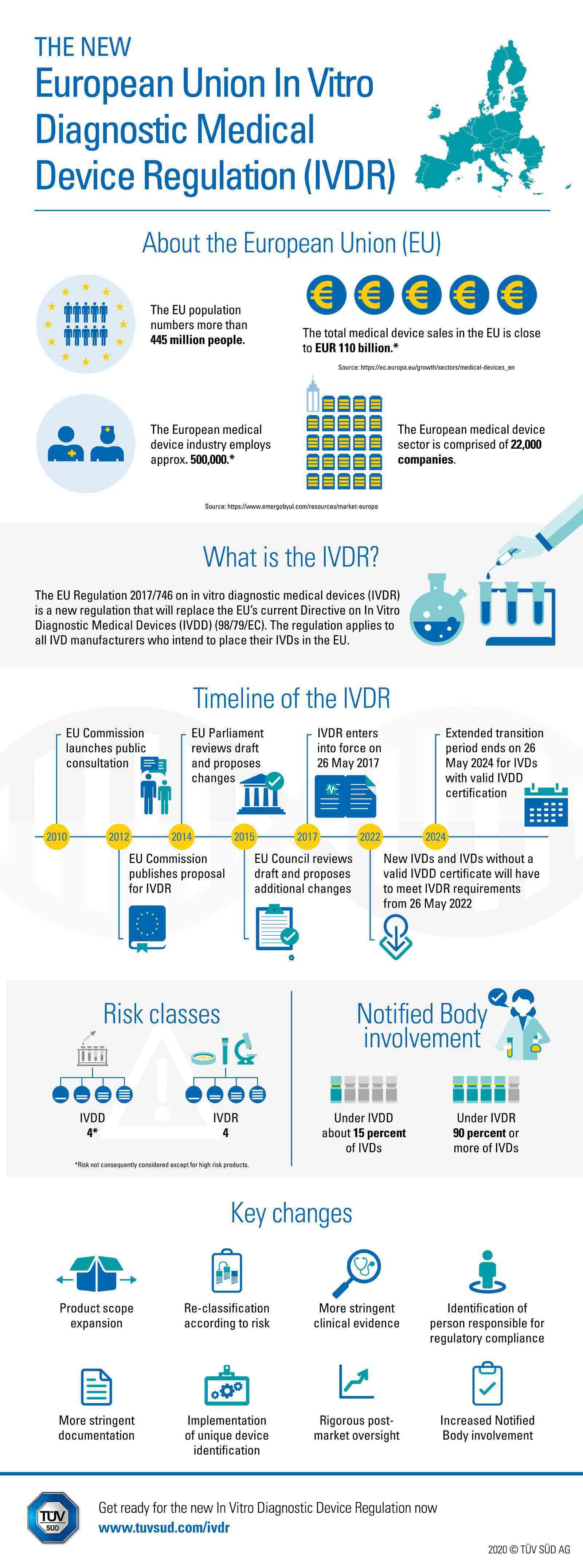
5 key implications from the new EU In-vitro Diagnostics Regulation — (Digital) Diagnostics Market Access & Policy Consulting based in Switzerland

/tuv-rheinland-ivdr-visual-1-en.png)