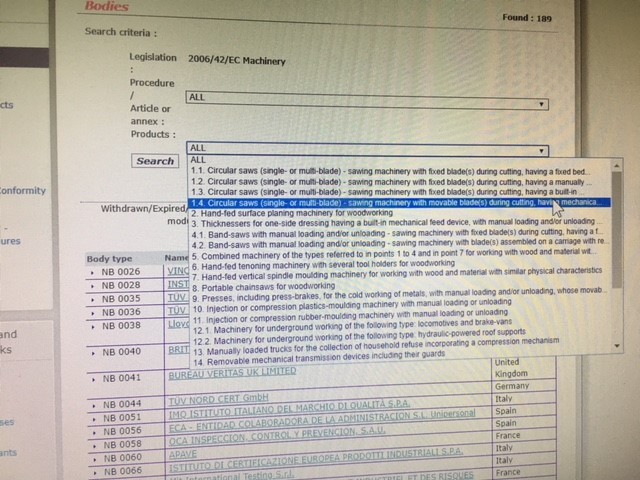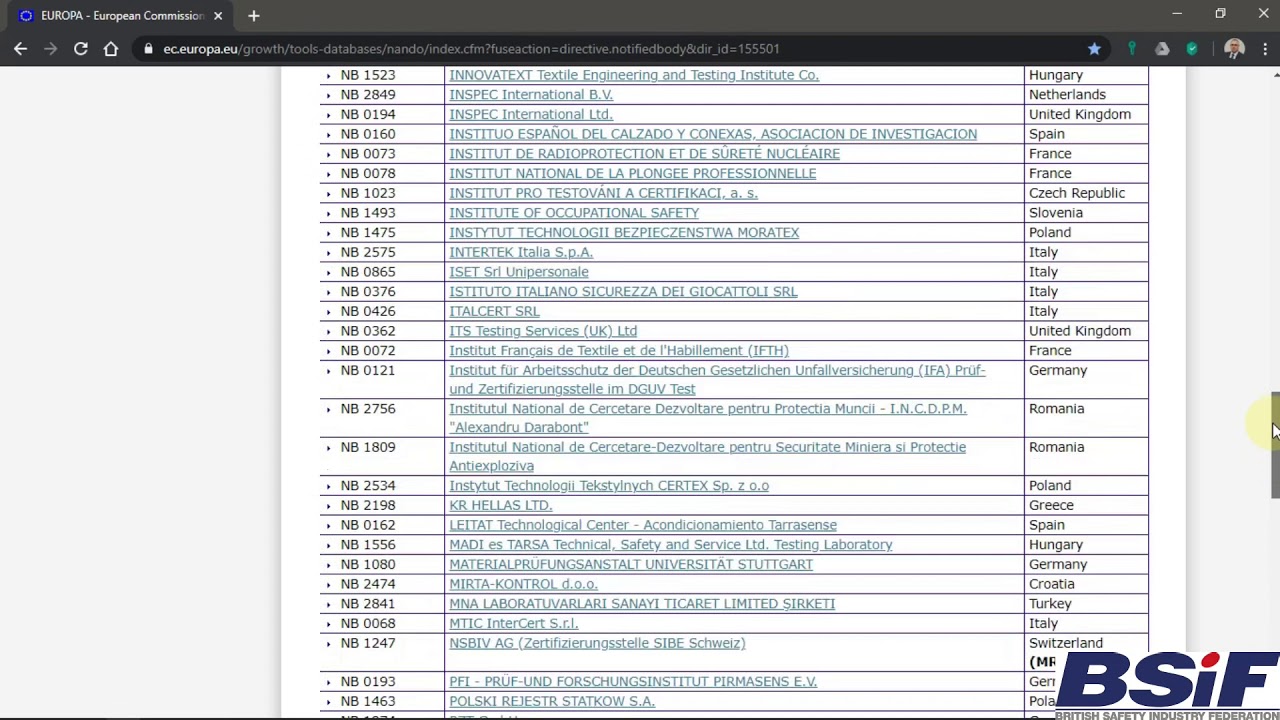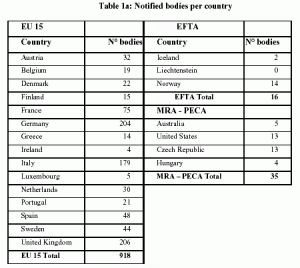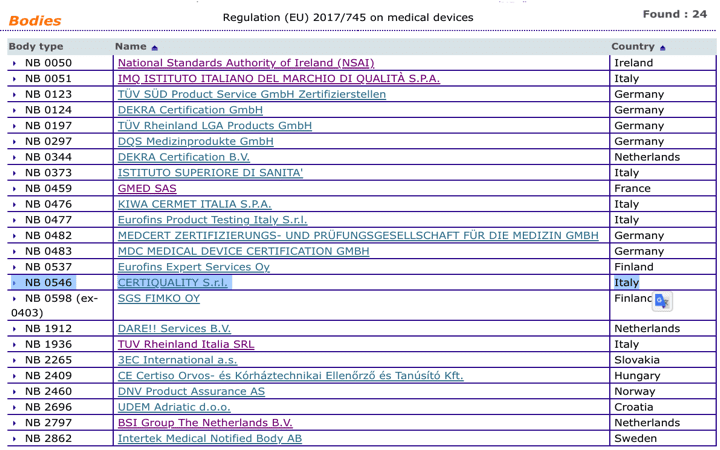
Organismos Notificados MDR (24): CERTIQUALITY (Italia) ON num. 0546 nuevo ON. Enhorabuena!!! | Red de Tecnologías Sanitarias y Productos Sanitarios

MDR: 26 Notified Bodies on NANDO & Swiss economic operator's requirements updated! · MDlaw – Information platform on European medical device regulations
Organismos Notificados MDR (41): UDEM (Turquia) ON num. 2292 nuevo Organismo Notificado. Enhorabuena !!!



![ARTICLE] Drug-led Combination Products: Checklist to get a Notified Body Opinion - Medidee Services ARTICLE] Drug-led Combination Products: Checklist to get a Notified Body Opinion - Medidee Services](https://medidee.com/wp-content/uploads/2023/01/Drug-led-combination-products-checklist.png)